Effect of a post-translational modification mimic on protein translocation through a nanopore
Abstract
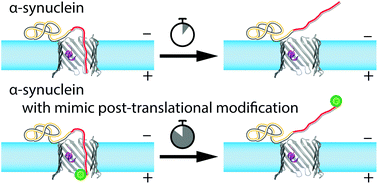
Post-translational modifications (PTMs) of proteins are recognized as crucial components of cell signaling pathways through modulating folding, altering stability, changing interactions with ligands, and, therefore, serving multiple regulatory functions. PTMs occur as covalent modifications of the protein's amino acid side chains or the length and composition of their termini. Here we study the functional consequences of PTMs for α-synuclein (αSyn) interactions with the nanopore of the voltage-dependent anion channel (VDAC) of the outer mitochondrial membrane. PTMs were mimicked by a divalent Alexa Fluor 488 sidechain attached separately at two positions on the αSyn C-terminus. Using single-channel reconstitution into planar lipid membranes, we find that such modifications change interactions drastically in both efficiency of VDAC inhibition by αSyn and its translocation through the VDAC nanopore. Analysis of the on/off kinetics in terms of an interaction “quasipotential” allows the positions of the C-terminal modifications to be determined with an accuracy of about three residues. Moreover, our results uncover a previously unobserved mechanism by which cytosolic proteins control β-barrel channels and thus a new regulatory function for PTMs.


 Please wait while we load your content...
Please wait while we load your content...