Nanocapillary confinement of imidazolium based ionic liquids
Abstract
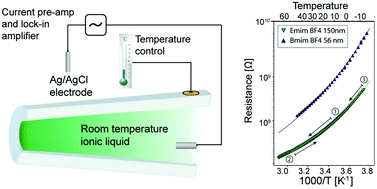
Room temperature ionic liquids are salts which are molten at or around room temperature without any added solvent or solution. In bulk they exhibit glass like dependence of conductivity with temperature as well as coupling of structural and transport properties. Interfaces of ionic liquids have been found to induce structural changes with evidence of long range structural ordering on solid–liquid interfaces spanning length scales of 10–100 nm. Our aim is to characterize the influence of confinement on the structural properties of ionic liquids. We present the first conductivity measurements on ionic liquids of the imidazolium type in single conical glass nanopores with confinements as low as tens of nanometers. We probe glassy dynamics of ionic liquids in a large range of temperatures (−20 to 70 °C) and nanopore opening sizes (20–600 nm) in silica glass nanocapillaries. Our results indicate no long range freezing effects due to confinement in nanopores with diameters as low as 20 nm. The studied ionic liquids are found to behave as glass like liquids across the whole accessible confinement size and temperature range.


 Please wait while we load your content...
Please wait while we load your content...