Efficient bifunctional catalysts for overall water splitting: porous Fe–Mo oxide hybrid nanorods†
Abstract
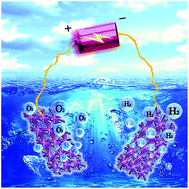
Efficient and inexpensive bifunctional catalysts for oxygen evolution reaction (OER) and hydrogen evolution reaction (HER) are essential for water splitting. Herein, we successfully prepare porous Fe–Mo oxide hybrid nanorods through a hydrothermal method followed by annealing at high temperature. They exhibit excellent catalytic activity for OER and HER in alkaline media, and produce a current density of 10 mA cm−2 at overpotentials of 200 and 66 mV. Besides, they work as bifunctional electrode materials for overall water splitting, achieving a current density of 10 mA cm−2 at a voltage of 1.52 V, and maintaining a current density of 60 mA cm−2 for 60 h. The unique morphology with self-supported structure can expose more active sites and facilitate charge transfer, and is not easy to peel off, thus it improves the catalytic activity and stability. This work therefore provides a valuable route for designing and fabricating inexpensive and high-performance catalytic materials for overall water splitting.


 Please wait while we load your content...
Please wait while we load your content...