Elucidating the capacitive desalination behavior of NaxCoO2: the significance of electrochemical pre-activation†
Abstract
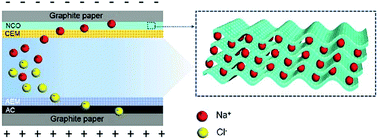
Hybrid capacitive deionization (HCDI) has emerged as a promising desalination technique due to its ultra-high salt removal capacity in high brine water. However, the mechanism behind HCDI is seldom discussed anywhere. Herein, we perform a comprehensive investigation to have some insight into the HCDI behavior of NaxCoO2 by varying x as 0.2, 0.5, 0.7, 1.0 and 1.6. Regardless of x, NaxCoO2 are classified as a representative P63/mmc space group with a P2 layered structure. With the increase of the sodium content, the (002) crystal plane of NaxCoO2 shifts significantly toward a high angle as the distance between CoO2 layers decreases. This results from the variation of the Na–O bonding length as well as the bonding energy according to the first-principles simulation. Moreover, it is observed that the Na–O bond broke once the input energy is higher than the Na–O bonding energy, leading to the electrochemical pre-activation of NaxCoO2. As a result, Na0.7CoO2 exhibits the best HCDI performance, i.e. a salt removal capacity of 63.0 mg g−1 and a charge efficiency of 97% in NaCl solutions with an initial conductivity of 2000 μS cm−1. Besides, the intercalation of sodium ions into NaxCoO2 has been confirmed by differentiating the respective contributions of pseudo-capacitance together with crystal phase transformation. Our results show that the desalination behavior of NaxCoO2 can be mediated by controlling the sodium content and electrochemical pre-activation.


 Please wait while we load your content...
Please wait while we load your content...