Novel polymeric micelles as enzyme-sensitive nuclear-targeted dual-functional drug delivery vehicles for enhanced 9-nitro-20(S)-camptothecin delivery and antitumor efficacy†
Abstract
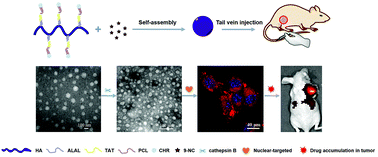
9-Nitro-20(S)-camptothecin (9-NC) is a broad-spectrum antitumor drug used in tumor treatments, but its clinical applications and antitumor efficacy are limited by its structural instability, poor solubility, and extremely low drug utilization in tumor tissues. In this study, enzyme-sensitive nuclear-targeted dual-functional polymeric micelles were developed for 9-NC delivery with a high drug loading content (12.93 ± 0.88%), steady-state circulation, and a rapid attack at the “heart” of tumor cells. Briefly, chrysin (CHR) as a π-conjugated moiety was immobilized on the PCL terminal in the TAT–PCL amphiphiles and combined with the ALAL peptide as a linker on HA chains to yield the ultimate CHR–PCL–TAT–ALAL–HA (HATPC) amphiphiles. Spherical 9-NC-loaded micelles were obtained from the self-assembly of the dual-functional amphiphiles comprising HATPC and 9-NC with uniform nanosize (121.6 ± 5.79 nm), well-distributed morphology (PDI: 0.256), and negative surface charge (−23.2 ± 0.5 mV), yielding high stability during blood circulation. In this drug delivery system, HA acts as an active tumor-targeting instrument via CD44-receptor-mediated endocytosis; further, the ALAL peptide could be cutoff in the lysosomes of the tumor cells due to the high expression of cathepsin B, leading to lysosomal escape, while the secondary polymeric micelles targeted the tumor cell nucleus via the exposed TAT peptide. The enzyme sensitivity and nuclei targetability of the 9-NC/HATPC micelles were confirmed by dynamic light scattering and confocal laser scanning microscopy analyses. As compared to free 9-NC and traditional mPEG2k–PCL2k polymeric micelles, 9-NC/HATPC micelles were the most concentrated in the tumor cell nucleus; therefore, they exhibited the highest cytotoxicity against SKOV3 tumor cells both in vitro (IC50 = 0.03 μg mL−1) and in vivo. This enzyme-sensitive nuclear-targeted dual-functional drug delivery system involving HATPC provided a new and promising strategy for enhanced 9-NC delivery and antitumor efficacy.


 Please wait while we load your content...
Please wait while we load your content...