Pyridine as a trigger in transformation chemistry from Au144(SR)60 to aromatic thiolate-ligated gold clusters
Abstract
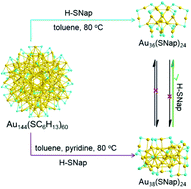
Transformation chemistry is a systematic methodology for achieving new atomically precise gold nanoclusters with specific physical and chemical properties. In this work, we have developed a new synthetic approach to prepare an aromatic thiolate-capped Au38(SNap)24 nanocluster via ligand exchange, size and structure transformation from the aliphatic thiolate-capped Au144(SC6H13)60 parent clusters triggered by the addition of a pyridine additive in the presence of excess 2-naphthalenethiol at thermal conditions (80 °C for 6 h). The Au38(SNap)24 nanoclusters have been well characterized by UV-vis spectroscopy and electrospray ionization mass spectrometry. The transformation pathway from Au144(SC6H13)60 to Au36(SNap)24 and Au38(SNap)24 undergoes different conversion pathways tailored by the pyridine additive in the etching system. Furthermore, the catalytic activity and selectivity of the Au cluster are largely influenced by the chemical nature of the protecting thiolate ligands in the Ullmann hetero-coupling reaction of iodobenzene and nitroiodobenzene. The aromatic ligands result in not only higher conversion but also remarkable increase in the selectivity toward the hetero-coupling product. The study provides new hints for the design and synthesis of new gold nanoclusters in transformation chemistry.


 Please wait while we load your content...
Please wait while we load your content...