Local mucosal immunization of self-assembled nanofibers elicits robust antitumor effects in an orthotopic model of mouse genital tumors†
Abstract
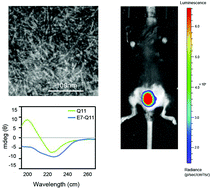
Human papillomavirus (HPV) is the identified causative agent of cervical cancer. Current therapeutic HPV vaccine candidates lack significant clinical efficacy, which can be attributed to insufficient activation of effector cells, lack of effective modification of the immunosuppressive tumor microenvironment, and the limitations of applied tumor models for preclinical vaccine evaluation. Here, a mouse model of orthotopic genital tumors was used to assess the effect of self-assembled nanofibers on eliciting a robust antitumor response via local mucosal immunization. A candidate vaccine was obtained by fusing HPV16 E744–62 to the self-assembling peptide Q11, which was assembled into nanofibers in a salt solution. Mice bearing an established genital TC-1 tumor were immunized with nanofibers through the intravaginal, intranasal, or subcutaneous route. Mucosal vaccination, especially via the intravaginal route, was more effective for suppressing tumor growth than subcutaneous immunization. The potential underlying mechanisms include promoting the systemic generation and tumor accumulation of antigen-specific cytotoxic T lymphocytes expressing high levels of interferon (IFN)-γ or granzyme-B, and reducing the tumor infiltration of immunosuppressive regulatory T cells and myeloid-derived suppressor cells. The levels of IFN-γ, the chemokines CXCL9 and CXCL10, and CXCR3+CD8+ T cells were significantly increased in tumor tissues, which may account for the improved recruitment of effector T cells into the tumor. Local mucosal immunization of nanofibers via the intravaginal route represents a new and promising vaccination strategy for the treatment of genital tumor lesions such as cervical cancer.


 Please wait while we load your content...
Please wait while we load your content...