Agaric-derived N-doped carbon nanorod arrays@nanosheet networks coupled with molybdenum carbide nanoparticles as highly efficient pH-universal hydrogen evolution electrocatalysts†
Abstract
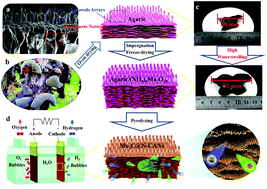
Non-precious, stable and efficient catalysts for the pH-universal hydrogen evolution reaction (HER) are highly desirable to meet the vast energy demands. Herein, we report a facile and scalable strategy using agaric as a precursor to construct a Mo2C-based HER electrocatalyst consisting of ultrafine Mo2C nanoparticles embedded within biomass-derived 3D N-doped carbon nanorod arrays@nanosheet networks (Mo2C@N-CANs). This electrocatalyst is highly active for the pH-universal hydrogen evolution reaction and requires overpotentials of only 82 mV, 100 mV and 350 mV to drive a current density of −10 mA cm−2 in acidic, alkaline and neutral media, exhibiting stable operation for 3000 cycles and 24 h long-term stability. Theoretical calculations indicate that coupling Mo2C, N and CANs into a hybrid results in producing wrinkles on carbon nanolayers, which changes the direction of sp2 hybrid orbitals to push the Gibbs free energy toward zero. This result reinforces the presence of a synergy effect between Mo2C and N-CANs in Mo2C@N-CAN catalysts, which leads to their impressive HER performances.


 Please wait while we load your content...
Please wait while we load your content...