Hot electron and thermal effects in plasmonic catalysis of nanocrystal transformation†
Abstract
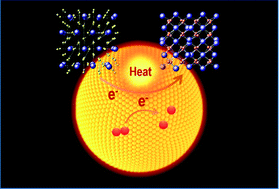
Plasmonic metal nanoparticles have the ability to harvest visible light and cause effective energy conversion, and they are considered as promising catalysts to drive chemical reactions. Although plasmonic catalysis has been widely used to mediate the reaction of organic molecules, the mechanism of contribution of thermal and hot carriers remains unclear. The catalysis of hot carriers is normally proposed as the dominant role of plasmonic catalysis, while the contribution of plasmonic thermal effects is often ignored, since the molecules on the metal surface are unstable at high temperatures. Here, plasmon catalytic nanocrystal transformation including oxidation reaction and optimization of the crystal structure is employed to investigate the plasmonic contributions of hot electron and thermal effects in plasmonic catalysis. It is found that the transformation rate and the corresponding product are very different with and without the assistance of hot electron catalysis. The thermal effect plays a dominant role in plasmon-catalyzed material transformation, and hot electrons can promote the oxidation reaction by facilitating the generation of active oxygen. The investigation provides insight into the specific role of hot electron and thermal effects in plasmonic catalysis, which is critically important for exploiting the highly localized fast plasmonic thermal effect and for designing energy-efficient plasmonic catalysts.


 Please wait while we load your content...
Please wait while we load your content...