The encapsulation of MnFe2O4 nanoparticles into the carbon framework with superior rate capability for lithium-ion batteries†
Abstract
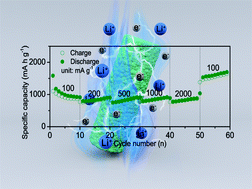
Binary transition metal oxides (BTMOs) have been regarded as one of the most hopeful anode materials for lithium-ion batteries (LIBs) owing to their high theoretical capacity, excellent electrochemical activity and abundant electrochemical reactions. However, BTMOs still suffer from two main problems, which are poor conductivity and large volume expansion during the charge/discharge processes. In order to address the above-mentioned problems, mesoporous MnFe2O4@C nanorods have been successfully synthesized in this work. The synergistic effect of the cross-linked carbon framework and mesoporous structure greatly improves the electrochemical performances. As expected, the mesoporous MnFe2O4@C electrode manifests discharge capacities of 987.5 and 816.6 mA h g−1 at the current densities of 100 and 2000 mA g−1, respectively, with the capacity retention ratio of 82.7%, exerting distinguished rate capabilities for LIBs.


 Please wait while we load your content...
Please wait while we load your content...