A bifunctional nanoporous Ni–Co–Se electrocatalyst with a superaerophobic surface for water and hydrazine oxidation†
Abstract
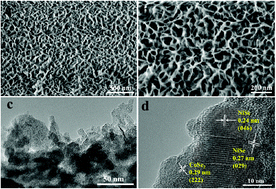
The sluggish kinetics of the oxygen evolution reaction (OER) has severely hindered the energetic convenience of water splitting. Thus, developing a highly efficient catalyst for the OER and replacing the OER with hydrazine oxidation (HzOR) are effective strategies for water electrolysis to achieve sustainable hydrogen production. Herein, bifunctional nanosheet arrays Ni0.6Co0.4Se with a porous structure were fabricated on Ni foam (NF) by the bubble dynamic template method during electrodeposition. Compared with CoSe2 and NiSe2, Ni0.6Co0.4Se exhibits excellent electrocatalytic performance for both the OER and HzOR. A low overpotential of only 249 mV is required to drive 10 mA cm−2, and a retention rate of nearly 100% after 24 h at 10 mA cm−2 is observed for Ni0.6Co0.4Se towards the OER. By substituting the OER by HzOR, an extremely high current density of 300 mA cm−2 at 0.4 V vs. RHE and a retention rate of 86.8% at 200 mA cm−2 after 12 h can be achieved. Interestingly, the mechanistic reason for the enhanced catalytic ability of Ni0.6Co0.4Se was studied, which is associated with the synergistic effects of Ni and Co, large ECSA, high electrical conductivity and most importantly the superaerophobic nature induced by the porous structure of Ni0.6Co0.4Se. The non-noble metal bifunctional electrocatalyst demonstrates a promising potential for application in both the OER and HzOR.


 Please wait while we load your content...
Please wait while we load your content...