Reversible cation exchange on macroscopic CdSe/CdS and CdS nanorod based gel networks†
Abstract
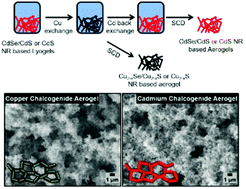
Over the past decades, cation exchange reactions applied to nanoparticles have opened up synthetic pathways to nanocrystals, which were not accessible by other means before. The limitation of cation exchange on the macroscopic scale of bulk materials is given by the limited ion diffusion within the crystal structure. Lyogels or aerogels are macroscopic, highly voluminous, porous materials composed of interconnected nanoscopic building blocks and hence represent a type of bridge between the macroscopic and the nanoscopic world. To demonstrate the feasibility of cation exchange on such macroscopic nanomaterials, the cation exchange on CdSe/CdS core/shell and CdS nanorod based lyogels to Cu2−xSe/Cu2−xS and Cu2−xS and the reversible exchange back to CdSe/CdS and CdS lyogels is presented. These copper-based lyogels can also be used as an intermediate state on the way to other metal chalcogenide-based macroscopic structures. By reversed cation exchange back to cadmium an additional proof is given, that the crystal structures remain unchanged. It is shown that cation exchange reactions can also be transferred to macroscopic objects like aerogels or lyogels. This procedure additionally allows the access of aerogels which cannot be synthesized via direct destabilization of the respective colloidal solutions.


 Please wait while we load your content...
Please wait while we load your content...