Direct observation of the geometric isomer selectivity of a reaction controlled via adsorbed bromine†
Abstract
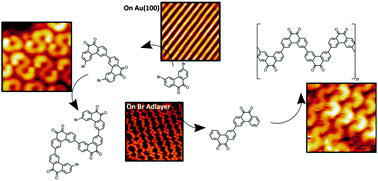
Methods to improve the specificity of stereoselective reactions are paramount to the viability of reaction-based processes. Surface-bound methods are a powerful means to carry out reactions with selectivity in the pursuit of specific products or nanoarchitectures through bottom-up assembly. The Ullmann-like coupling reaction has come to represent one of the most useful methods to form two-dimensional structures through covalent couplings of aromatic molecules following the dissociation of an aryl carbon-halide bond. The leaving halogen atoms are proven to remain adsorbed on the surface and can be deleterious to the fabrication of larger conjugated superstructures. However, on Au(100) we have found the leaving halogen atoms generate a new adsorbate surface that leads to geometric isomer selectivity compared to the unmodified metal surface. The covalent coupling of 3,6-dibromo-phenanthrenequinone (DBPQ) was studied and leaving bromine atoms were found to form self-assembled islands and modify the reconstruction of Au(100). Subsequently, the coupling reaction yielded total selectivity towards a radical trans dimer when surrounded by bromine atoms, while only cis dimers were observed on the undecorated Au surface. This selectivity induced by bromine networks on the surface ultimately results in another potent way to control the stereoselectivity of surface-bound coupling reactions.


 Please wait while we load your content...
Please wait while we load your content...