Expanding the structural diversity of peptide assemblies by coassembling dipeptides with diphenylalanine†
Abstract
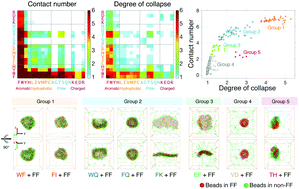
Molecular self-assembly is a bottom-up approach to fabricate novel supramolecular structures. While the structural diversity obtained by the use of a single type of building block is limited, coassembly of different peptides has recently evolved as an extended strategy to expand the diversity of peptide nanoarchitectures. Here we systematically investigate the coassembly of diphenylalanine (FF) with each one of the 399 non-FF dipeptides by micro-second molecular dynamics simulations. Our simulations show that dipeptides, by coassembling with FF, display a greatly enhanced aggregation propensity and a significantly expanded structural diversity. Regular-shaped vesicles, single- or multi-cavity assemblies, and planar sheets are formed by coassembly of FF with different types of non-FF dipeptides, which are rarely observed in self-assemblies of non-FF dipeptides. Interaction analyses reveal that the formation of these varied structures is attributed to a delicate balance between aromatic stacking, hydrophobic, and electrostatic repulsion interactions. This study provides structural and mechanistic insights into the coassembly of FF and non-FF dipeptides, thus offering a possible way to achieve a controllable design of bionanomaterials through FF-involved dipeptide coassembly.
- This article is part of the themed collection: Theoretical Modelling at Nano-bio Interfaces


 Please wait while we load your content...
Please wait while we load your content...