Effective replacement of cetyltrimethylammonium bromide (CTAB) by mercaptoalkanoic acids on gold nanorod (AuNR) surfaces in aqueous solutions†
Abstract
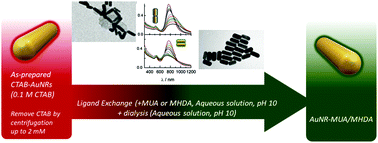
The highly packed cetyltrimethylammonium bromide (CTAB) bilayer built up on the surface of gold nanorods (AuNRs) when synthesized by the seed-mediated procedure hampers the complete ligand exchange under experimental conditions that preserves the stability of the dispersions. In the present work, a ligand exchange protocol by using carboxy-terminated alkanethiols of different chain lengths by means of a green approach that uses only aqueous solutions is presented. The protocol is based on the knowledge of the stability in the aqueous solution of both the starting CTAB-AuNRs and the final products that help in the choice of the experimental conditions used for ligand exchange. The characterization of the CTAB protective layer as well as the study of its colloidal stability in solution has helped us to design an appropriate methodology. Cyclic voltammetry of CTAB-AuNRs demonstrates the high stability of the bilayer showing the existence of a two-dimensional phase transition from a highly ordered to a less organized phase. Other techniques such as XPS, FT-IR and Raman spectroscopy provide information about the structure of the layer and UV-visible-NIR spectroscopy establishes the stability conditions in aqueous solution. We have chosen an exchange procedure for 11-mercaptoundecanoic acid (MUA) and 16-mercaptohexadecanoic acid (MHDA) based on a one-pot methodology under conditions where all the species involved are stable. The protocol, however, can be extended to different chemical functionalities that are considered useful to be applied in living systems. Under these conditions the complete exchange of CTAB by the mercaptoderivatives was successful as demonstrated by the different characterization techniques used: UV-visible-NIR, FT-IR, Raman, XPS spectroscopy, cyclic voltammetry and transmission electron microscopy (TEM).

 Please wait while we load your content...
Please wait while we load your content...