Single-atom transition metals supported on black phosphorene for electrochemical nitrogen reduction†
Abstract
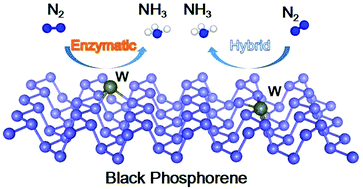
The electrochemical nitrogen reduction reaction (NRR) is one of the most promising routes to produce ammonia under mild conditions. Black phosphorene (BP) has attracted wide attention as an NRR electrocatalyst owing to its high Fermi level and unique electronic structure. However, the low intrinsic activity of surface sites greatly restricts its application in the electrochemical NRR. In this work, we theoretically designed a series of single-atom transition metals anchored on the BP surface with MP3 (M = Fe, Mn, Cr, Mo, W, V and Nb) active sites for the NRR via density functional theory (DFT) calculations. By taking stability, activity and selectivity into consideration, the single-atom W-anchored BP was selected as a promising candidate for the NRR. The energy-favorable enzymatic pathway on W@BP (W atoms adsorb on the surface of BP) and the hybrid pathway on W–BP (W atoms substitute the surface P atoms of BP) have reaction onset potentials of 0.46 and 0.42 V, respectively, indicating that the single-atom W-anchored BP shows high activity towards the NRR. This high performance originates from the WP3 active sites, which act as an electron adaptor to activate N2 by donating electrons, thereby greatly regulating the charge transfer between BP and the reaction intermediates. This study proposes a promising active catalyst and provides theoretical guidance to construct BP-supported transition metal single-atom electrocatalysts for the NRR.
- This article is part of the themed collection: Editor’s Choice: Computational studies of nanomaterials for energy, catalysis and electronics


 Please wait while we load your content...
Please wait while we load your content...