An Fe–V@NiO heterostructure electrocatalyst towards the oxygen evolution reaction†
Abstract
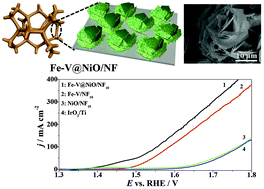
The development of a nonprecious and Earth-abundant electrocatalyst with high electrocatalytic activity for the oxygen evolution reaction (OER) is an emerging hot issue and remains a grand challenge. In the present work, we proposed a facile strategy to construct ultrathin NiO nanosheets decorated with Fe–V nanoparticles on nickel foam (Fe–V@NiO/NF) for use as an OER electrocatalyst. Due to the 3D rational configuration, the Fe–V@NiO/NF with a heterostructure shows excellent electrocatalytic activity towards the OER. Interestingly, it is found that in situ oxidation by galvanostatic electrolysis in alkaline solution is beneficial to enhance the OER performance. After 10 h of electrolysis, a current density of 50 mA cm−2 is achieved at a low overpotential of 271.1 mV. This is because during the in situ oxidation process, iron and vanadium ions insert into the NiO lattice and lead to the generation of highly active α-FeOOH and an amorphous (oxy)-hydroxide layer. Additionally, the charge transfer resistance dramatically reduces with the prolonging of oxidation time.


 Please wait while we load your content...
Please wait while we load your content...