Homogeneous nucleation of Li2O2 under Li–O2 battery discharge†
Abstract
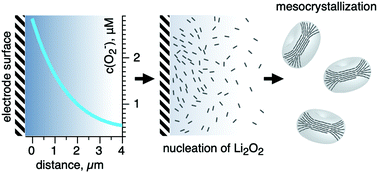
The development of high-energy lithium–oxygen batteries has significantly slowed by numerous challenges including capacity limitations due to electrode surface passivation by the discharge product Li2O2. Since the passivation rate and intensity are dependent on the deposit morphology, herein, we focus on the mechanisms governing Li2O2 formation within the porous cathode. We report evidence of homogeneous nucleation of Li2O2 crystallites and their further assembly in bulk of the electrolyte solution in DMSO, which possesses a high donor number. After careful estimation of the superoxide ion concentration distribution within a phenomenological model, it was found that the high stability of superoxide ions formed during the ORR towards disproportionation and sufficient diffusivity of (0.5–1.2) × 10−6 cm2 s−1 enabled Li2O2 nucleation and crystallization not only at the surface but also in the electrolyte, and the reaction zone spread throughout the internal space of the porous electrode. High initial supersaturation promoted the homogeneous nucleation of Li2O2 nanoplates, which instantly assembled into mesocrystals also in the solution bulk. These results were supported by operando SAXS/WAXS and morphology observations. Thus, although homogeneous nucleation is not dominant, it is important for achieving a high capacity in Li–O2 batteries.


 Please wait while we load your content...
Please wait while we load your content...