On the mechanism of reduction of M(H2O)mn+ by borohydride: the case of Ag(H2O)2+†
Abstract
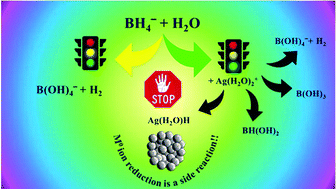
The redox potentials of M(H2O)mn+/M0(atom) couples are often far too negative to enable the formation of M0(atom) by most reducing agents. Therefore, one has to reconsider the mechanism of formation of M0-NPs by the bottom-up procedure. A deep and detailed theoretical analysis of the reduction of Ag(H2O)2+ by BH4− points out that silver cations act mainly as catalysts of the reactions BH4− + 4H2O → B(OH)4− + 4H2. However, the transition states of the catalyzed process differ from those of the un-catalyzed process. The formation of (H2O)Ag–H, which is the starting stage for the formation of intermediates with Ag–Ag bonds, is only a side reaction in the process. Experimental evidence of the complexity of the process is presented, by stopped-flow; at least four processes are observed prior to the formation of Ag0-NPs. The spectra of these intermediates differ from those of Ag0atom and Ag2+aq. Though DFT calculations were performed only for silver cations, it is believed that analogous mechanisms are involved in the reductions of other cations.


 Please wait while we load your content...
Please wait while we load your content...