Entropic effect and residue specific entropic contribution to the cooperativity in streptavidin–biotin binding†
Abstract
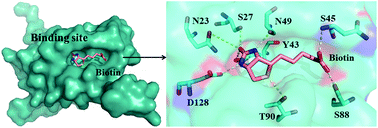
Molecular dynamics (MD) simulations were performed employing the polarized protein-specific charge (PPC) to explore the origin of the cooperativity in streptavidin–biotin systems (wild type, two single mutations and one double-mutation). The results of the experiment found that the existence of cooperativity is mainly the result of the entropic effect. In this study, the entropic contribution to the binding free energy was calculated using the recently developed interaction entropy (IE) method, and computational results are in excellent agreement with the experimental observations and are further verified by the calculation of the thermodynamic integration. Comparison of different force fields in terms of predicted binding strength ordering, cooperativity of energy and the stability of hydrogen bonding suggests that the PPC force field combined IE method is a suitable choice. In addition, the IE method enables us to obtain the residue-specific entropic contributions to the streptavidin–biotin binding affinity and identify ten hot-spot residues providing the dominant contribution to the cooperative binding. Importantly, the overall cooperativity obtained from the ten residues also comes mainly from the entropic effect in our study. The calculation of the potential of mean force shows that the unbinding of streptavidin–biotin is a multi-step process, and each step corresponds to the formation and rupture of the hydrogen bond network. And S45A mutation may increase the rigidity of the linker region, making the flap region relatively difficult to open. The present study provides significant molecular insight into the binding cooperativity of the streptavidin–biotin complex.


 Please wait while we load your content...
Please wait while we load your content...