Dehydration reactions in polyfunctional natural products
Abstract
Covering: up to 2020
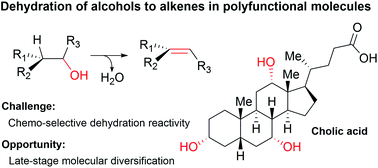
In this review, we present state of the art methods for performing dehydration reactions in alcohol substrates to deliver alkene products. The dehydration of alcohols typically proceeds through activation of the alcoholic moiety to a nucleofugal species followed by a subsequent elimination step. While the alcohol is a quintessential functional group, selective dehydration of alcohols in complex molecular scaffolds has not been harnessed to allow molecular diversification strategies. We present the perspective of utilizing complex molecular compounds containing alcoholic functionalities to generate novel molecular constructs that impose on chemical space of characterized bioactivity. Nature inspires the direct and selective dehydration of alcohols in complex molecules and demonstrates a potential that has not yet been realized by chemical methodology. We present challenging substrates for direct and selective dehydration reactions and argue that chemical methodology solving the challenges presented will be valued by synthetic and natural product chemists alike.


 Please wait while we load your content...
Please wait while we load your content...