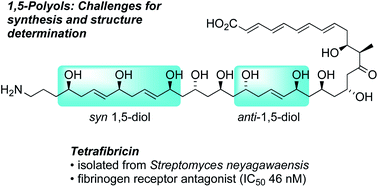
Inspirations from tetrafibricin and related polyketides: new methods and strategies for 1,5-polyol synthesis
Abstract
Covering: up to 2019
Selective synthesis with control of remote stereogenic centers has long been a challenge in organic chemistry. In recent years the interest in this topic has been energized by isolation and synthetic studies of tetrafibricin and other natural products containing 1,5-polyols, such as amphidinol 3, marinomycins, and caylobolide. Here we discuss recent developments in 1,5-polyol synthesis, including an overview of selected bioactive natural products in this class and examples of new synthetic methodologies and strategies dedicated to remote stereocontrol in these structures. To illustrate in greater depth, we review several instructive examples of how these innovations have been applied in synthetic studies on tetrafibricin.


 Please wait while we load your content...
Please wait while we load your content...