Enantioselective formation of quaternary carbon stereocenters in natural product synthesis: a recent update
Abstract
Covering: 2013–2018
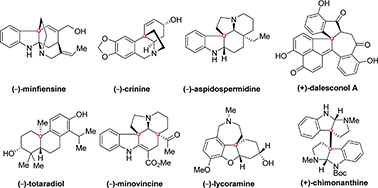
Natural products bearing quaternary carbon stereocenters have attracted tremendous interest from the synthetic community due to their diverse biological activities and fascinating molecular architectures. However, the construction of these molecules in an enantioselective fashion remains a long-standing challenge because of the lack of efficient asymmetric catalytic methods for installing these motifs. The rapid progress in the development of new-generation efficient chiral catalysts has opened the door for several asymmetric reactions, such as Michael addition, dearomative cyclization, polyene cyclization, α-arylation, cycloaddition, allylation, for the construction of quaternary carbon stereocenters in a highly enantioselective fashion. These asymmetric catalytic methods have greatly facilitated the synthesis of complex natural products with improved output and overall efficiency. In this concise review, we highlight the progress in the last six years in complex natural product synthesis, in which at least one quaternary carbon stereocenter has been constructed via asymmetric catalytic technologies, with particular emphasis on the analysis of the stereochemical model of each enantioselective transformation.


 Please wait while we load your content...
Please wait while we load your content...