Structures and magnetic properties of novel Ln(iii)-based pentanuclear clusters: magnetic refrigeration and single-molecule magnet behavior†
Abstract
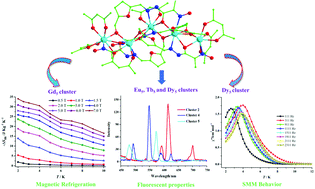
Six new and novel polynuclear LnIII5 clusters, formulated as [Ln5(L)4(acac)7]·solvents (Ln(III) = Sm (1), Eu (2), Gd (3), Tb (4), Dy (5) and Ho (6), Hacac = acetylacetone, and H2L = 2-[(2-(hydroxyimino)propanehydrazide)methyl]-5-hydroxymethyl-2-furancarboxylic acid) have been successfully obtained through the reaction of a polydentate Schiff base ligand (H2L) and Ln(acac)3·2H2O. Single crystal X-ray diffraction data indicate that clusters 1–6 are isostructural and their central Ln(III) ions have a Ln(III)5 chain arrangement. Magnetic property investigations show that the Gd(III)-based cluster (3) exhibits a significant and larger MCE with the maximum −ΔSm value of 34.02 J kg−1 K−1 (ΔH = 7.0 T and T = 2.0 K). Alternating current (ac) susceptibility measurements of the Dy(III)-based cluster (5) display remarkable slow magnetic relaxation behaviors at zero applied direct current (dc) magnetic field (ΔE/kB = 17.47 K and τ0 = 1.52 × 10−6 s). Furthermore, studies on the solid-state fluorescence properties show that clusters 2, 4, and 5 display the characteristic lanthanum luminescence at room temperature.


 Please wait while we load your content...
Please wait while we load your content...