Surface engineering of silica nanoparticles with a gadolinium–PCTA complex for efficient T1-weighted MRI contrast agents†
Abstract
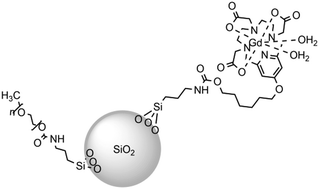
New pyridine containing triAza; 3,6,9,15-tetraazabicyclo[9.3.1]pentadeca-1(15),11,13-triene-3,6,9-triacetic acid (PCTA) ligands presenting pendant carboxylic acid or alcohol functions have been synthesized and used to form diaqua Gd(III) complexes, which have been immobilized onto dense silica nanoparticles. Cytotoxicity studies of these complexes (free or immobilized on the nanoparticles) on normal 1BR3G cells and cancerous HCT 116 cells indicated a dependence on the pendant chemical group for the free complexes. However, this effect disappeared once the complexes were immobilized on the surface of the nanoparticles, leading to non-toxic nanomaterials. The interest of these complexes (free or immobilized on dense silica nanoparticles) as contrast agents in magnetic resonance imaging (MRI) has been evaluated in comparison with DOTAREM® by recording their nuclear magnetic resonance dispersion (NMRD) profiles, measuring their transversal and longitudinal relaxivities as well as recording images on phantoms at 37 °C. This study has evidenced the high potential of these complexes: first it suggested the possibility to reduce the dose to be injected by a factor of 10, second it evidenced their high efficiency in high field T1-weighted MRI (9.4 T), the key towards images of higher resolution and shorter acquisition times.


 Please wait while we load your content...
Please wait while we load your content...