Synthesis of 6-substituted indoloquinazolinones from arynes and 2-acyl-4-quinazolinones: a transition-metal free C–N and C–C bond formation strategy†
Abstract
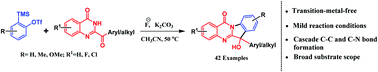
A versatile transition-metal free synthetic strategy has been developed for the direct synthesis of 6-substituted indoloquinazolinones from 2-acyl-4-quinazolinones and aryne precursors under mild conditions. This cascade strategy proceeds via successive C–N and C–C bond formation in a single reaction vessel by simple treatment with CsF and K2CO3. A diverse range of 6-substituted indoloquinazolinones were synthesized in good yields with excellent functional group tolerance.


 Please wait while we load your content...
Please wait while we load your content...