Porphyrazines with annulated diazepine rings. 5. Near-IR-absorbing tetrakis(6,7-dihydro-1H-1,4-diazepino)porphyrazines and effects of acid solvation on their spectral properties†
Abstract
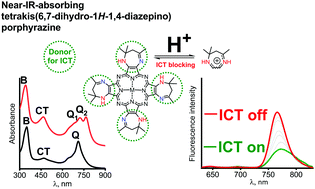
Novel near-IR-absorbing porphyrazines, tetrakis-(5,7,7-trimethyl-6,7-dihydro-1H-1,4-diazepino)porphyrazines (Me12Dz4PzMgII and Me12Dz4PzH2) and their cyclohexyl derivatives (Cy8Dz4PzMgII and Cy8Dz4PzH2), were prepared by template cyclotetramerization of the corresponding 6,7-dihydro-1H-1,4-diazepine-2,3-dicarbonitriles in the presence of magnesium or lithium butoxides. The resulting macrocycles were characterized using IR, MALDI TOF, 1H NMR, and UV-Vis-spectroscopy and cyclic voltammetry. The presence of the electron acceptor imino and donor secondary amino groups in the fused 6,7-dihydro-1H-1,4-diazepine rings leads to strong polarization of the π-chromophore and shifts the Q band maxima in the near IR region (710–740 nm for the MgII complexes, and 750–770 nm for the metal free macrocycles). The NH-groups in the diazepine fragments participating in intramolecular charge transfer (ICT) strongly quench the fluorescence. Spectrophotometric titration, 1H NMR measurements and quantum chemical molecular modeling indicate that protonation of the diazepine rings which occurs on the imino nitrogen atoms switches off the ICT effect and increases the fluorescence quantum yields by 2–4 times.


 Please wait while we load your content...
Please wait while we load your content...