Synthesis, cytotoxic activity, ADMET and molecular docking study of quinoline-based hybrid compounds of 1,5-benzothiazepines†
Abstract
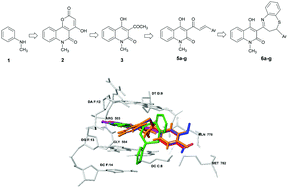
Some α,β-unsaturated ketones 4a–g of 3-acetyl-4-hydroxyquinolin-2(1H)-one were prepared by its reaction with (hetero)aromatic aldehydes with yields of 61–87% using piperidine as a catalyst. These ketones reacted with o-aminothiophenol in the presence of acetic acid to afford a series of new hybrid compounds, quinoline-benzothiazepine, 6a–g. The yields of benzothiazepines 6a–g were 62–85%. All the synthesized compounds 6a–g were screened for their in vitro anticancer activity against human hepatocellular carcinoma HepG2 and squamous cell carcinoma KB cancer lines. Compounds 6d and 6g had the best activity in the series, with IC50 values of 0.25 and 0.27 μg mL−1, respectively, against HepG2, and of 0.26 and 0.28 μM, respectively, against KB cell lines. ADMET properties showed that compounds 6c and 6g possessed drug-likeness behavior. Cross-docking results indicated that residues GLN778(A), DA12(F), and DG13(F) in the binding pocket were potential ligand binding hot-spot residues for compounds 6c and 6g.


 Please wait while we load your content...
Please wait while we load your content...