2-Hydroxyimino-6-aza-pyrimidine nucleosides: synthesis, DFT calculations, and antiviral evaluations†
Abstract
The global public health concerns and economic impact caused by emerging outbreaks of RNA viruses call for the search for new direct acting antiviral agents. Herein, we describe the synthesis, DFT calculations, and antiviral evaluation of a series of novel 2-hydroxyimino-6-aza-pyrimidine ribonucleosides. DFT//B3LYP/6-311+G** calculations of the tautomeric distributions of the 2-hydroxyimino nucleosides 7, 8, and 9 in aqueous environments indicate a predominance of the canonical 2-(E)-hydroxyimino structure, where the hydroxyl group points away from the sugar moiety. The conformer distributions of the latter geometrical isomers of 7, 8, and 9 support the formation of five membered rings via hydrogen bonding between the (E)-C2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–O–H moiety and N3–H of 7 and 8 and between (E)-C2
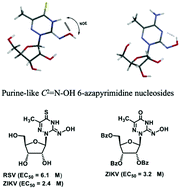
N–O–H moiety and N3–H of 7 and 8 and between (E)-C2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–O–H and N3 of 9, creating purine shaped nucleosides with the glycosidic linkage at the pyrimidine ring. The newly synthesized nucleosides were screened against an RNA viral panel, of which moderate antiviral activity was observed against Zika virus (ZIKV) and human respiratory syncytial virus (HRSV). 6-Aza-2-hydroxyimino-5-methyluridine derivative 18 showed activity against ZIKV (EC50 3.2 μM), while its peracetylated derivative 19 showed activity against HRSV (EC50 5.2 μM). The corresponding 4-thiono-2-hydroxyimino derivative 8 showed activity against HRSV (EC50 6.1 μM) and against ZIKA (EC50 2.4 μM). This study shows that the 6-aza-2-hydroxyimino-5-methyluracil derived nucleosides can be further optimized to provide potent antiviral agents.
N–O–H and N3 of 9, creating purine shaped nucleosides with the glycosidic linkage at the pyrimidine ring. The newly synthesized nucleosides were screened against an RNA viral panel, of which moderate antiviral activity was observed against Zika virus (ZIKV) and human respiratory syncytial virus (HRSV). 6-Aza-2-hydroxyimino-5-methyluridine derivative 18 showed activity against ZIKV (EC50 3.2 μM), while its peracetylated derivative 19 showed activity against HRSV (EC50 5.2 μM). The corresponding 4-thiono-2-hydroxyimino derivative 8 showed activity against HRSV (EC50 6.1 μM) and against ZIKA (EC50 2.4 μM). This study shows that the 6-aza-2-hydroxyimino-5-methyluracil derived nucleosides can be further optimized to provide potent antiviral agents.


 Please wait while we load your content...
Please wait while we load your content...