Modulation of oxygen functional groups and their influence on the supercapacitor performance of reduced graphene oxide
Abstract
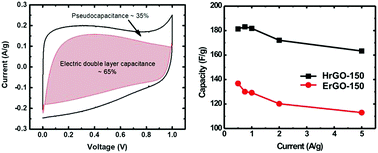
The capacitive properties of graphene-based supercapacitor electrode materials are closely related to their geometric structure and chemical nature. Their geometric structure has been well studied, however, the physical and chemical mechanisms giving rise to the conductivity and capacitance of the oxygen functional group configurations are still unclear. In this study, using water or ethanol as the solvent, reduced graphene oxide with a similar total oxygen content but different oxygen function groups was synthesized, which provided a platform to reveal the mechanism. The results show that the oxygen functional groups that are doubly bound to aromatic carbon make a greater contribution to the conductivity, while the oxygen functional groups that are singly bound to aliphatic carbon and singly bound to aromatic carbon make a greater contribution to the supercapacitance. The specific capacitance of reduced graphene oxide using water as the solvent can reach 182 F g−1, which is much higher than the value for reduced graphene oxide using ethanol as the solvent (136 F g−1). The capacitive behavior, including the electric double layer capacitive behavior and pseudocapacitive behavior, were studied systematically. This study demonstrates that through controlling the oxygen functional groups, the performance of graphene based supercapacitors can be further improved.


 Please wait while we load your content...
Please wait while we load your content...