Inverse sandwich complexes of B7M2−, B8M2, and B9M2+ (M = Zr, Hf): the nonclassical M–M bonds embedded in monocyclic boron rings†
Abstract
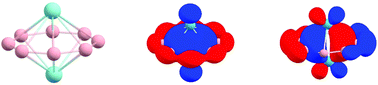
A number of doped boron clusters with metals have been achieved with intriguing electronic and structural properties. In this work, we found the inverse sandwich complexes of B7M2−, B8M2, and B9M2+ (M = Zr, Hf) to be a global minimum, where the M2 motif perpendicularly embeds in the B7, B8, and twist B9 monocyclic boron rings. The interactions between M2 and boron rings, including the dp–π interactions and the electrostatic interactions originating from the charge transfer from the M to the boron rings, provide driving forces that stabilize such title complexes. The effective charge transfer gives rise to perfectly delocalized (3π + 3σ) orbitals, leading to double aromaticity of the whole species, especially when the three delocalized σ orbitals of the boron rings are perpendicularly mixed with one negligible σ and two π bonds of the M2 motifs, giving rise to less pronounced and nonclassical bonding interactions between two short-contact M atoms.


 Please wait while we load your content...
Please wait while we load your content...