Planar tetracoordinate carbon molecules with 14 valence electrons: examples of CBe4Mnn−2 (M = Li, Au; n = 1–3) clusters†
Abstract
Planar tetracoordinate or pentacoordinate carbon (ptC and ppC) systems are dominated by the 18-electron counting, and a 14-electron ptC cluster is generally considered odd if not impossible. Herein we report computational evidence for a series of ternary CBe4Mnn−2 (M = Li, Au; n = 1–3) clusters, which feature 14 valence electrons and a uniform ptC center. These global-minimum clusters are established via computer global searches, followed by electronic structure calculations at the PBE0-D3, B3LYP-D3, and single-point CCSD(T) levels. In all six species, a core ptC CBe4 unit is stabilized by one to three peripheral Li/Au atoms in a bridging fashion. Chemical bonding analyses reveal delocalized 2π/6σ frameworks around the ptC center, thus rendering double π/σ aromaticity according to the (4n + 2) Hückel rule. The delocalized π/σ frameworks collectively conform to the 8-electron counting and are irrelevant to the “18-electron rule”. The 14-electron ptC clusters can potentially be extended to isoelectronic CBe4Mnn−2 (M = Na, K, Cu, Ag; n = 1–3) systems. Cationic ptC CBe4M3+ (M = Li, Na, K, Cu, Ag, Au) clusters have low vertical electron affinities (2.36–4.77 eV) and belong to the class of exotic species called superalkali or pseudoalkali cations.
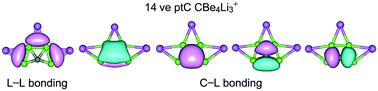


 Please wait while we load your content...
Please wait while we load your content...