The fixation of CO2 by epoxides over nickel-pyrazolate-based metal–organic frameworks†
Abstract
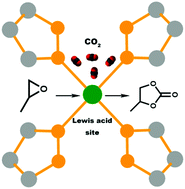
CO2 emissions reduction is one of the major concerns of human beings. The cycloaddition of CO2 and an epoxide to form a cyclic carbonate is a promising reaction for reducing atmospheric CO2 while also producing valuable industrial chemicals. In this work, we developed a series of Ni-pyrazolate-based metal–organic framework (MOF) catalysts, Nibdp-X (X = H and NO2) and Ni3btp2, to catalyze this reaction under solvent-free conditions. Herein, bdp and btp are 1,4-(4-bispyrazolyl)benzene and 1,3,5-tris(1H-pyrazol-4-yl)benzene, respectively. The unsaturated metal centers (or Lewis acid sites) in the MOFs play a key role in the cycloaddition of CO2 and epoxide. The catalytic performance of the catalysts is significantly influenced by the density and accessibility of Lewis acid sites. Moreover, the recyclability of the MOF catalysts was studied as well.


 Please wait while we load your content...
Please wait while we load your content...