Ultra-small iridium nanoparticles as active catalysts for the selective and efficient reduction of nitroarenes†
Abstract
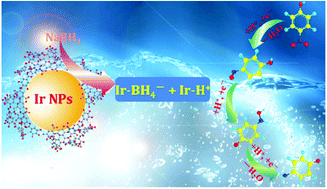
Ultra-small noble metal iridium nanoparticles (IrNPs) possessing superior catalytic activity were prepared using tannin as a stabilizer via a controlled one-step synthesis. Their average size was about 3.5 ± 0.5 nm, and they were enclosed with high-index facets {200} and {311}. They possessed high catalytic activity and could be applied in the efficient and selective catalytic reduction of nitroarenes under mild reaction conditions for the first time. The catalytic reduction rates of different nitroaromatic compounds including 4-nitrophenol (p-NP), p-nitrotoluene (p-NT), p-nitroaniline (p-NA), 4-nitrobenzoic acid (p-NBA) and 4-nitrochlorobenzene (p-NCB) were compared to investigate the reduction mechanism of the IrNPs. The results indicate that catalytic reduction rates were in the order p-NBA > p-NCB > p-NT > p-NP > p-NA. The nitroarenes with electron-withdrawing substituents can be reduced faster than those the electron-donating substituents, suggesting that an electron transfer mechanism was involved in the system of nitroaromatic compound-NaBH4-nanomaterials.


 Please wait while we load your content...
Please wait while we load your content...