A series of benzothiazole-based Schiff bases for the colorimetric sensing of fluoride and acetate ions: acetate-induced turn-on fluorescence for selectivity†
Abstract
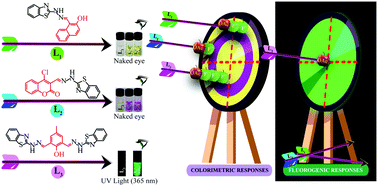
Three benzothiazole-functionalized Schiff bases have been synthesized and applied as colorimetric sensors for fluoride and acetate ions in acetonitrile. The absorption spectroscopy-based sensing aptitude of L1, [(E)-1-((2-(benzo[d]thiazol-2-yl)hydrazineylidene)methyl)naphthalen-2-ol] has been enhanced by introducing a coumarin moiety in L2, [(E)-3-((2-(benzo[d]thiazol-2-yl)hydrazineylidene)methyl)-4-chloro-2H-chromen-2-one]. Colorimetric response of L2 is better than L1 with rapid naked eye detection of both fluoride and acetate ion via a palpable change in colour and 147 nm red shift. The selectivity between the two anions is accomplished by using emission spectroscopy with another Schiff base L3. Owing to its dimeric cage-like structure, L3 can selectively sense an acetate ion based on its shape complementarity and emitting turn-on fluorescence at 520 nm in the presence of the aforementioned ion. L2 exhibits the lowest detection limit for fluoride ions with a limit of detection as low as 3.35 × 10−8 M via colorimetric responses, while L3 detects acetate ions down to 2.907 × 10−8 M with a turn-on fluorescence signal. 1H NMR studies confirm the sensing mechanism to be the initial formation of hydrogen bonds with –OH and –NH protons, and the subsequent deprotonation of the most acidic protons upon addition of excess fluoride and acetate ions.


 Please wait while we load your content...
Please wait while we load your content...