Probing the structural evolution, electronic and spectral properties of beryllium doped magnesium and its ion clusters†
Abstract
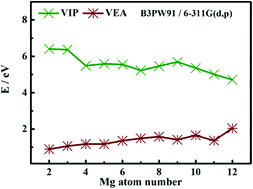
Beryllium doped small-sized magnesium and its ion clusters are fully studied in this work. CALMPSO software was used to search for BeMgQn (Q = 0, ±1, n = 2–12) clusters’ structures. The structural optimization with density functional theory (DFT) of BeMgQn clusters was performed by two functionals B3PW91/6-311G(d,p) and PBE0/6-311G(d,p). Although the structures of clusters are diverse, their geometries with 3D structures can be summarized as two types of growth mechanism based on tetrahedron and tower-like. Calculations of the size dependence of the lowest energy state of BeMgQn clusters using the two functionals, including average binding energy, second-order energy difference, HOMO–LUMO energy gap, VIP and VEA, coincidentally indicate that BeMg09, BeMg+19 and BeMg−18 are the local most stable clusters. For providing theoretical guidance for future experimental observations, the theoretical computations and simulations of BeMg−1n clusters’ PES and BeMgQn (Q = 0, ±1, n = 2–12) clusters’ IR and Raman spectra were performed. Finally, ELF, MO and DOS analyses of the local most stable clusters were carried out, and it was found that their stability mainly originated from the σ-type covalent bond formed by the interaction between the s- and p-orbitals of Be and Mg atoms.


 Please wait while we load your content...
Please wait while we load your content...