Formation of ammonium ions by electrochemical oxidation of urea with a boron-doped diamond electrode†
Abstract
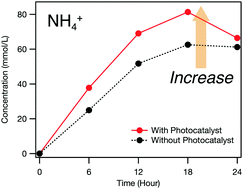
Ammonium ions were formed by electrochemical oxidation of urea with a boron-doped diamond (BDD) electrode. Almost complete decomposition of urea was achieved. When the BDD electrode was used together with a mesoporous titanium dioxide photocatalyst, the amount of ammonium ions produced increased.


 Please wait while we load your content...
Please wait while we load your content...