Engineering of an archaeal phosphodiesterase to trigger aggregation-induced emission (AIE) of synthetic substrates†
Abstract
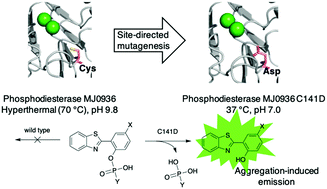
Aggregation-induced emission (AIE) probes that can be triggered by enzymatic activity are sought for applications across the life sciences. The compound 2-(2-hydroxyphenyl)benzothiazole (HBT) is non-fluorescent in solution but exhibits AIE. To adapt HBT for potential clinical applications, two phosphoester derivatives (1 and 2) were prepared. For extension to molecular brachytherapy (delivery of a radionuclide), one phosphoester derivative (3) was prepared wherein a bromine atom is incorporated as a site for subsequent radioiodide substitution. Each probe 1–3 requires phosphatase action to engender AIE. For specificity in diagnostic and therapeutic applications, a heterologous (i.e., foreign to humans) enzyme was sought for conversion of 1–3 from aqueous-soluble to aqueous-insoluble form. Here, an archaeal phosphodiesterase PDE-MJ0936 (wild-type) was systematically engineered for activity toward 1–3. PDE-MJ0936 is a hyperthermal enzyme (activity at 70 °C and pH 9.8) and exists as a dimer, which together limit life science applications. Ten PDE mutants were engineered by site-directed mutagenesis to achieve a monomeric structure, wider substrate scope, and elastic substrate docking under physiological conditions. Among the mutants, PDE-C141D exhibited efficient activity towards bis(4-nitrophenyl)phosphate (Km = 0.28 mM, kcat = 3.0 s−1 and kcat/Km = 1.1 × 104 M−1 s−1) and efficiently hydrolyzed 3 at 37 °C and pH 7.0, conditions under which PDE-MJ0936 is silent. The substrates 1–3 are relatively resistant to alkaline phosphatase, PDE-MJ0936 and fetal bovine serum. The development of enzymatically triggered substrates and the complementary heterologous enzymes may support diverse applications in the life sciences.


 Please wait while we load your content...
Please wait while we load your content...