N-(Cyano(naphthalen-1-yl)methyl)benzamides: synthesis, crystal structures, and colorimetric sensing of fluoride anions†
Abstract
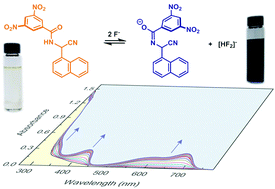
A series of N-(cyano(naphthalen-1-yl)methyl)benzamide derivatives were synthesized through direct acylation reactions of 2-amino-2-(naphthalen-1-yl)acetonitrile. The solid-state properties of these compounds were revealed by X-ray single crystallography, while their hydrogen bonding interactions in the solution phase were examined through UV-Vis absorption and NMR analyses. Among these benzamide derivatives, the compound that contains a 3,5-dinitrophenyl group was found to exhibit a drastic color transition from colorless to achromatic black in response to fluoride anion at mM concentrations, attesting to its excellent performance in terms of naked-eye detection of fluoride anion in solution. The colorimetric sensing behavior was attributed to a deprotonation-enhanced intramolecular charge transfer (ICT) mechanism based on UV-Vis and NMR titration experiments in conjunction with density functional theory (DFT) calculations.


 Please wait while we load your content...
Please wait while we load your content...