Mechanistic insights into the allylic oxidation of aliphatic compounds by tetraamido iron(v) species: A C–H vs. O–H bond activation†
Abstract
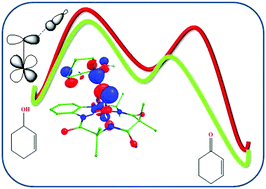
The main challenges in synthetic organic chemistry are chemoselective oxidation of C–H bonds by natural complexes under mild conditions. Heme and non-heme metal complexes with several oxidants such as dioxygen, hydrogen peroxide, m-chloroperbenzoic acid etc. are used for the catalytic oxidation of aliphatic C–H bonds. Metal catalyzed allylic oxidation of aliphatic compounds results in attractive intermediates and these are very useful in pharmaceutical industries. Here we have reported electronic structures and also for the first time the mechanistic detail of selective allylic oxidation of cyclohex-2-enol by an oxidant non-heme iron oxo species using hybrid density functional theory incorporating dispersion effects. Our DFT results show that a significant spin density on the ferryl oxygen plays an important role in activating O–H and C–H bonds of the cyclohex-2-enol. The reaction can be feasible via O–H (pathway a) and C–H (pathway b) bond activation. The initial hydrogen abstraction is the rate-determining step in both the pathways. A significant spin density on carbon and oxygen atoms shows that O–H/C–H bond activation occurs through homolytic cleavage. DFT investigation shows that the O–H bond activation of the studied mechanism can show two-state reactivity whereas C–H bond activation can proceed via single state reactivity. By mapping energy profiles, we have found that the C–H bond activation is relatively favored over the O–H and oxygen attack. Furthermore, the significant exchange of metal electrons and structural parameters during transition states can control reactivity and can help to design catalysts with better efficiency/selectivity for catalytic reactions.


 Please wait while we load your content...
Please wait while we load your content...