Supramolecular organization and optical properties of BODIPY derivatives in Langmuir–Schaefer films†
Abstract
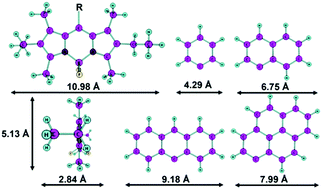
Four BODIPY (boron-dipyrrin) dyes with various condensed aromatic moieties (phenyl, naphthyl, anthryl and pyrenyl) as 8-substituents were chosen and their supramolecular organization and properties in Langmuir–Schaefer (LS) films with different numbers of layers were investigated. The spectral characteristics of the dyes in LS-films and solution and the structure of the films were examined. The BODIPY substituent structure influences the character of the intermolecular interactions of the dyes in LS-films. The phenyl-substituted compound was found to form excimers with a long wavelength fluorescence peak at 582 nm upon the layer number increase, yet only minor bathochromic shifts of the peak position were observed for the films of the other investigated compounds. Moreover, unlike in liquid solutions, the dyes in LS-films were not found to exhibit solvatochromic properties. All of the compounds (except anthryl-substituted) were found to retain fluorescence in solid films. Transfer of films from a floating monolayer was indicated by further material analysis: the measured average periodicity corresponds to a single-layer structure of BODIPY molecules located parallel to the surface with an interplanar distance of d = 0.3 nm. The obtained results broaden the prospects of BODIPY based thin film material applications, denoting versatile modification routes leading to increased fluorescence, tunable excimer formation equilibrium and crystallinity.


 Please wait while we load your content...
Please wait while we load your content...