Molecular modelling of mebendazole polymorphs as a potential colchicine binding site inhibitor
Abstract
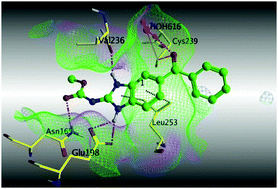
Preclinical and clinical studies on mebendazole revealed its potential as an anticancer agent. We therefore aimed to investigate its binding interactions with one of the most important cancer targets (the tubulin protein) depending on its structural similarity with the original co-crystallized ligand (nocodazole), besides characterization of the electronic configuration at the molecular level. By reviewing the binding mode and hydrogen bond lengths between the three polymorphs of mebendazole (MBZ) and the colchicine binding site on the tubulin protein, form B of MBZ is the form expected to bind more efficiently with the tubulin protein among the other forms. The calculated physicochemical properties revealed also that form B is the most lipophilic form, and hence can more flawlessly cross the blood brain barrier in order to target brain tumors. Our study has ramifications to consider form B of MBZ in clinical trials of repurposing MBZ in oncology, because only forms A and C have been considered while form B was abandoned.


 Please wait while we load your content...
Please wait while we load your content...