Iron(ii) coordination pyrazole complexes with aromatic sulfonate ligands: the role of ether†
Abstract
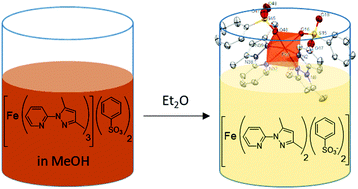
Four novel heteroleptic mononuclear Fe(II) coordination complexes including 3,5-dimethyl-1-(2′-pyridyl)-pyrazole (DMPP) and aromatic sulfonate ligands were synthesized and characterized using single crystal-XRD, SQUID magnetometry and 57Fe Mössbauer spectroscopy. The crystal structures of the complexes revealed the unusual coordination of sulfonate groups to Fe(II) ions, leading to an Fe–N4O2 coordination environment. This specific molecular layout is presumably caused by diethyl ether, which was vapour diffused into the complexation medium to obtain crystalline complexes. Alongside yielding single crystals, diethyl ether also altered the self-assembly process of a usual FeN6 homoleptic coordination environment. All the complexes remain paramagnetic at all temperatures as suggested by magnetic susceptibility measurements. All are stable in air except [Fe(DMPP)2(tos)2] which got partially oxidized to Fe(III) over time as suggested by 57Fe Mössbauer spectroscopy.


 Please wait while we load your content...
Please wait while we load your content...