On the planarity of the cyclobutane ring in the crystal of dimethyl 2,4-bis(3,4-dimethoxyphenyl)cyclobutane-1,3-dicarboxylate: a natural bond orbital and Hirshfeld surface analysis study†
Abstract
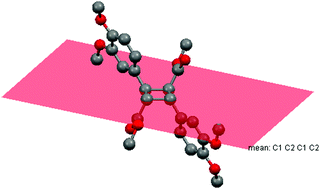
Dimethyl 2,4-bis(3,4-dimethoxyphenyl)cyclobutane-1,3-dicarboxylate was synthesized by the dimerization of methyl 3-(3,4-dimethoxyphenyl)acrylate in methanol under photoirradiation by direct sunlight. Its molecular and crystal structures were determined by single crystal X-ray analysis. The molecule is located at the center of inversion. Thus, the asymmetric unit contains only one half of the molecule, and the central four-membered ring is in a slightly distorted square-planar arrangement. The residues on opposite sides of the four-membered ring are located on different faces of the ring, resembling the typical α-form for truxillic acid derivatives. The analysis of natural bond orbital population was performed and the effect of the σC–H → σC–H* hyperconjugative interactions is determined. In the crystal structure, the C–HMthcarbx⋯OCarbx and C–HMthoxy⋯OMthoxy [Mthcarbx = methylcarboxylate, Carbx = carboxylate and Mthoxy = methoxy] hydrogen bonds, enclosing R22(12) and R22(18) ring motifs, link the molecules into a three-dimensional architecture, in which they may be effective in the stabilization of the structure. The Hirshfeld surface analysis of the crystal structure confirms that the most important contributions for the crystal packing are from H⋯H (51.8%), H⋯O/O⋯H (31.5%) and H⋯C/C⋯H (15.3%) interactions. Hydrogen bonding and van der Waals interactions are dominant interactions in crystal packing.


 Please wait while we load your content...
Please wait while we load your content...