Mechanistic insights can resolve the low reactivity and selectivity issues in intermolecular Rauhut–Currier (RC) reaction of γ-hydroxyenone†
Abstract
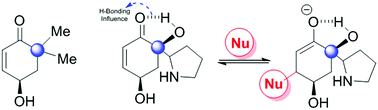
Dimerization or isomerization of electron-deficient alkenes in the presence of organophosphine catalysts involves the Rauhut–Currier (RC) reaction. In this study, we attempt to understand the reaction pathway and propose a mechanism based on the evidence from computational simulations. The long-term goal is to establish the RC reaction as a general way of constructing complex natural products by dimerization. We used two selected substrates, dimethyl hydroxyacetone and hydroxyl pyrrolidinyl hydroxyacetone in the present study. The challenges to overcome are the lack of reactivity and selectivity. We determined that the issue of low reactivity can be resolved by shifting the equilibrium of the enone/enolate forms of the substrate towards the enolate.


 Please wait while we load your content...
Please wait while we load your content...