Synthesis of the first 2-hydroxyanthraquinone substituted cyclotriphosphazenes and their cytotoxic properties†
Abstract
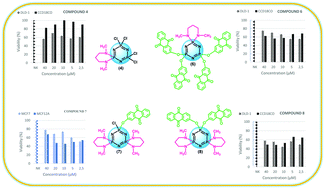
Breast cancer, which is common in women, is the second most common cause of cancer-related deaths after lung cancer. Colon cancer has the highest mortality rate and the fourth highest incidence in the world. Many new anticancer candidate compounds are synthesized for cancer treatment. However, since the side effects of these compounds have unfortunately not yet been remedied, new drugs need to be developed. Anthraquinone derivatives, one of this class of compounds, are important to cancer researchers because they can inhibit cell proliferation in some common cancers. In this current study, novel 2-hydroxyanthraquinone based cyclotriphosphazenes (6–9) have been prepared for the investigation of anti-cancer activities. The compounds were evaluated for cytotoxicity by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay to four different cells: MCF-7 (human breast adenocarcinoma), MCF-12A (normal breast epithelium), DLD-1 (human colon adenocarcinoma) and CCD-18Co (normal colon epithelium). Notable activity of compounds 4 and 6, the activity of compound 8 was observed against the DLD-1 cell line, activity of compound 7 was observed against the MCF-7 cell line via IC50 values of 5 μM and 2.5 μM. Encouragingly, the IC50 values of 40 μM to normal cells were also acquired for compounds 4 and 8 against the DLD-1 cell line, and compound 7 against the MCF-7 cell line, which show the low cytoxicity and appropriate selectivity indices of the compounds.


 Please wait while we load your content...
Please wait while we load your content...