An efficient synthesis of chloro-aminocyclooctanediol and aminocyclooctanetriol: an unexpected acetolysis product†
Abstract
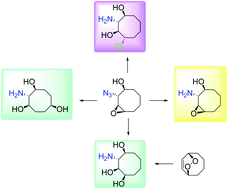
2-Azidocyclooct-3-en-1-ol was used as a key compound for the synthesis of various eight-membered ring aminocyclitols. The double bond was subjected to an epoxidation reaction for further functionalization. The azidoepoxide obtained was subjected to a ring-opening reaction with HCl(g) in methanol. 2-Amino-4-chlorocyclooctanediol was synthesized as a single isomer in high yield. Benzylation of the hydroxyl group in 2-azidocyclooct-3-en-1-ol followed by an epoxidation reaction, acetolysis of the epoxide ring, and benzyl deprotection resulted in the unexpected formation of two azido-cyclooctanetriol isomers. Subsequently, hydrogenation of the azide group in these isomers afforded 3-amino- and 2-aminocyclooctanetriols. The mechanism of the formation of the products is discussed. Epoxidation of cyclooctene endoperoxide, which was the second key compound, followed by hydrogenation and azidolysis of epoxy-diol provided the target 3-aminocyclooctanetriol as the sole product.


 Please wait while we load your content...
Please wait while we load your content...