Aerobic oxidation of 2-aminophenol catalysed by a series of mononuclear copper(ii) complexes: phenoxazinone synthase-like activity and mechanistic study†
Abstract
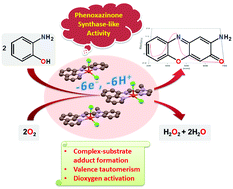
Three mononuclear copper(II) complexes of types [Cu(L1)(Cl)2]·MeOH (1·MeOH), [Cu(L2)(Cl)2]·H2O (2·H2O) and [Cu(L3)(Cl)2] (3) have been synthesized from three reduced Schiff base tridentate N3 ligands, namely N-(pyridin-2-ylmethyl)quinolin-8-amine ([H2]L1), N-(1-methylbenzimidazol-2-ylmethyl)quinolin-8-amine ([H2]L2), and N-(1-methylimidazol-2-ylmethyl)quinolin-8-amine ([H2]L3), respectively, having variable donor moieties. During metalation all three reduced Schiff base ligands undergo oxidative dehydrogenation in situ under aerobic conditions to yield the corresponding Schiff base ligated mononuclear copper(II) complexes. All complexes have been characterized using various spectroscopic techniques such as IR, HRMS-ESI, UV-vis, and EPR. Structural characterization of each complex by single crystal X-ray diffraction reveals that the coordination environment around the copper ion is distorted square pyramidal. The three complexes effectively catalyse the aerial oxidation of 2-aminophenol (H2AP) to 2-amino-phenoxazine-3-one (APX), thus mimicking the catalytic function of the enzyme phenoxazinone synthase. Kinetic studies have been done to arrive at the following catalytic efficiency order: 3 ≫ 2·H2O > 1·MeOH. The observed trend can be explained by considering the structure–function relation of the catalytic activity. Intramolecular charge distribution (valence tautomerism) within a complex–substrate adduct leads to the generation of a “CuI–(substrate radical)” tautomer. This phenomenon has been established by EPR spectroscopy, particularly using 2-anilino-4,6-di-tert-butylphenol (H2APPh,t-Bu, a structural analogue of H2AP) as a substrate. Such a “CuI–(substrate radical)” species is believed to promote dioxygen activation. The effects of temperature and pH on the reaction rates have been studied. Activation parameters (Ea, ΔH‡, and ΔS‡) have been evaluated from temperature-dependent kinetic measurements. A plausible reaction pathway has been proposed on the basis of stoichiometry determination, spectroscopic data and kinetic analysis.


 Please wait while we load your content...
Please wait while we load your content...