A new approach to the mechanism for the acetalization of benzaldehyde over MOF catalysts†
Abstract
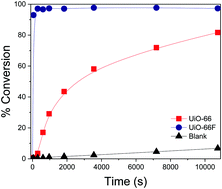
The benzaldehyde acetalization reaction catalyzed by UiO-66, and its fluorinated analog UiO-66F, was carried out in a batch-type reactor at room temperature and atmospheric pressure, and the full kinetic study was performed using the Langmuir–Hinshelwood and Eley–Rideal models. It was established that the Eley–Rideal model is the one that best fits the experimental data. The catalytic results indicated that both MOFs enable carrying out the acetalization reaction. However, UiO-66F has the highest activity, which could be attributed to its high acidity. Both structures were characterized by N2 physisorption, thermogravimetry, powder X-ray diffraction, potentiometric titrations, and infrared spectroscopy. The highest acidity displayed by UiO-66F was explained by DFT studies and experimental studies.


 Please wait while we load your content...
Please wait while we load your content...