Synthesis, photophysical and electropolymerization properties of thiophene-substituted 2,3-diphenylbuta-1,3-dienes†
Abstract
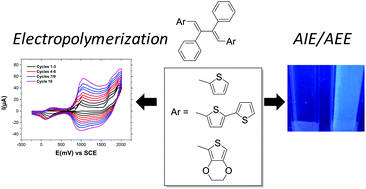
A series of 2,3-diphenylbuta-1,3-dienes (DPBs) bearing thiophene (T-DPB), bithiophene (BT-DPB) and ethylenedioxythiophene (EDOT-DPB) electropolymerizable units were prepared through desilylation reactions of the corresponding 3,4-diphenylsilole derivatives. These DPB derivatives exhibit remarkably different aggregation induced emission (AIE) or aggregation enhanced emission (AEE) behaviour depending on the strength of the molecular interactions occurring in the solid state. Indeed, T-DPB and EDOT-DPB were found to be good AIEgens while BT-DPB exhibited AEE behaviour. Finally, the electrochemical properties of these new materials were investigated revealing for all DPBs the occurrence of electropolymerization processes leading potentially to low band gap polymers.


 Please wait while we load your content...
Please wait while we load your content...